The biggest enhancement of shut aseptic packaging devices is definitely the institution of a sterile place. The sterilization of packaging paper is performed in a very hydrogen peroxide tub inside the sterile room, reducing the necessity for just a wetting agent and enhancing the security of aseptic functions.
Secondary packaging techniques are gaining prominence as a result of growing need for strong security and Improved branding. Corrugated boxes, shrink wraps, and custom cartons competently safeguard goods in the course of transit and storage. Corporations utilize visually attractive models that elevate model recognition and client engagement.
Pakona also serves the construction marketplace by furnishing entire plants for production bolstered concrete pipes. The organization values each individual customer marriage and is dedicated to offering excellent service and aid.
If the opened vacant bag moves on the posture underneath the sound material hoppers, it's filled with substance or injected with liquid substance by a liquid feeder.
The hydrogen peroxide made use of right here ought to incorporate a wetting agent to decrease the area tension with the hydrogen peroxide.
Get the job done with companies which offer staff members instruction to enhance devices use. In addition, validate the availability of well-informed customer guidance teams who will swiftly take care of difficulties. By prioritizing sturdy immediately after-gross sales assistance, you ensure smooth functions, minimizing disruptions and maximizing productiveness in your packaging procedures.
Automated packaging techniques present several advantages. They increase productivity by growing packaging speed and reducing human error. These methods assure consistency, giving uniform packaging that satisfies good quality requirements. By lowering the necessity for guide labor, businesses preserve on operational expenditures.
Making sure that the sterile space is just not contaminated by microorganisms, sterile liquid packaging machines air is constantly released to maintain optimistic stress all through manufacturing. The horizontal and longitudinal sealing on the shut aseptic packaging program is comparable to that of the open up system.
Digital parts Digital components that happen to be sensitive to electrostatic costs for example wafers, chips, motherboards and printed circuit boards can be packaged safely, liquid packaging machines freed from dust, Dust and moisture. A Henkelman prepared to get the job done within an ESD Harmless setting is ideal fitted to this.
Meanwhile, Superior sensors guarantee quality Regulate by detecting problems in actual-time. These innovations not only boost operational effectiveness but in addition lead considerably to sustainable procedures by decreasing waste and Power usage. Because the industry evolves, integrating these cutting-edge technologies turns into essential for manufacturers to stay aggressive and environmentally responsible.
Gross sales Division is chargeable for communicating purchaser's prerequisites with various department for complete technique
[5] In the seventies MA offers achieved the shops when bacon and fish were being sold in retail packs in Mexico. Because then development has become continuous and fascination in MAP has developed on account of shopper demand from customers.
Our fillers and movies may be made use of independently of one another; nonetheless, we propose that they be used with each other. When built-in, our auto fillers and films realize the speediest processing speeds within the field, with unique benefits furnished by the complete Taisei Lamick program.
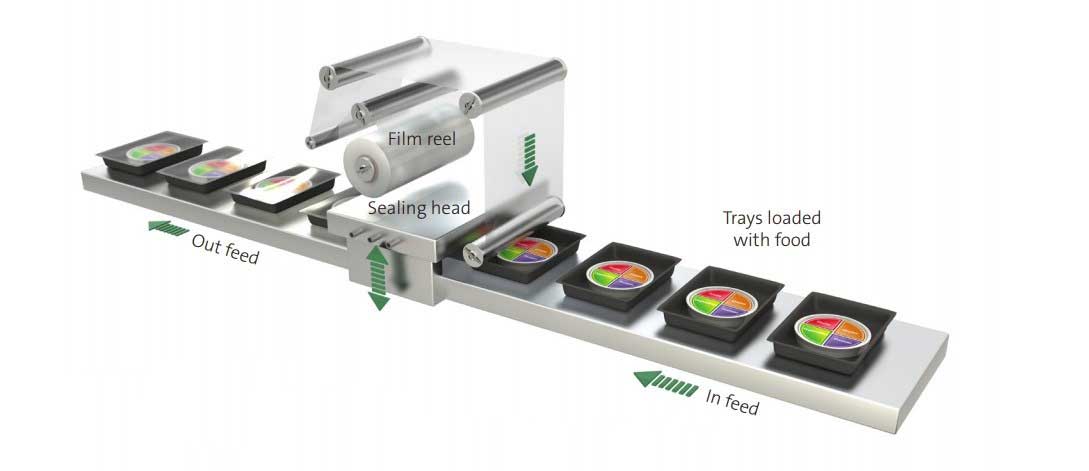
Figure 5-40 displays a typical closed aseptic packaging technique. In manufacturing, the packaging paper rises with the paper roll to your longitudinal sealing strip applicator, where the longitudinal strip is heat-sealed to at least one side from the packaging paper, then enters the hydrogen peroxide bathtub for sterilization. The sterilized packaging paper sorts a paper cylinder In the sterile area.
 Val Kilmer Then & Now!
Val Kilmer Then & Now! Christina Ricci Then & Now!
Christina Ricci Then & Now! Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now! Gia Lopez Then & Now!
Gia Lopez Then & Now! Terry Farrell Then & Now!
Terry Farrell Then & Now!